In relation to the medical and clinical testing of our devices, we hereafter give you the results of 11 different (medical) organizations who have tested our MedicCleanAir® Mobile HEPA/ULPA Units and have published these results in reports of which some world-wide by esteemed Medical Organizations such as the Hospital Infection Society and the Bone Marrow Transplant. Additional information and details of the test reports can be obtained at the MedicCleanAir® office.
Report HumanVision Augenklinik Zürich 2019
Operating theatre
Objective of the test / installation: to obtain ISO7 with installation of 2x COMBI devices
Results
Particle count gives ISO7 classification. CFU count 0. No fungi detected
Report University Hospital Brussels – Belgium – 2018
Operating theatre nr. 14
The operating theatre is equipped with a laminar flow system that gives ISO6 under the plenum, ISO7 around the plenum. Objective of the test / installation: to create ISO6 in all areas of the operating theatre.
Results
After implementation of the MedicCleanAir COMBI, the operating theatre was classified as ISO6 for particle count as well as for CFU count. No fungi detected.
Report University Hospital Brussels – Belgium – 2018
Operating theatre nr. 7
The operating theatre is equipped with a laminar flow system that gives ISO6 under the plenum, ISO6 around the plenum. Objective of the test / installation: to create ISO5 in all areas of the operating theatre.
Results
After implementation of the MedicCleanAir COMBI, the operating theatre was classified as ISO5 for particle count as well as for CFU count. No highly contagious fungi was detected.
Report Santa Croce Hospital Cuneo Italy – University of Genoa – National Cancer Institute Italy.
This organization wanted to know what MedicCleanAir® Mobile HEPA Units were capable of on a long term basis – 2 YEARS OF TESTING – for immune compromised patients.
The following documents can be found in topic ‘press’:
- 1 a) S. Croce e Carle Cuneo – Italy BMT 2002
- 1 b) same organization, but then a cost/benefit analysis which states that a yearly savings of € 161'000.– is being made by implementing MedicCleanAir®.
Triparametric Study on Aspergillosis
clinical – laboratory – microbiological
Department Bone Marrow Transplant
University Genoa / S.Croce Cuneo
Conclusion
MedicCleanAir® Mobile HEPA Units reduce not only the contamination of the patient rooms, the ward did NOT HAVE ANY INFECTED PATIENT SINCE THE INSTALLATION OF MEDICCLEANAIR® MOBILE HEPA UNITS!
Report University Hospital Antwerp Belgium – Neonatology Department: effect of MedicCleanAir® Mobile HEPA Units on the contamination in the NICU ward during renovation works
This organization wanted to know what MedicCleanAir® Mobile HEPA Units were capable of in reducing contamination due to renovation works taking place in a neonatology intensive care unit. – during 1 year of monitoring –
The following document can be found in topic ‘press’:
- Journal of Hospital Infections 2000
Drawing
Conclusion
MedicCleanAir® Mobile HEPA Units reduce contamination with at least 75% over all, and between 90 and 100% in the isolation rooms. The MedicCleanAir® Mobile HEPA Units were put – as per manufacturer’s instructions – at level 2.
Report University Hospital Bonn Germany – Hematology Department: effect of MedicCleanAir® Mobile HEPA Units on contamination within the patient rooms
This organization wanted to know if the MedicCleanAir® Mobile HEPA Units could constantly maintain the level of ZERO CFU/m3 for a long period of time, even when windows are often opened in the patient room.
The following documents can be obtained at the office:
- 3 a) DGKH Germany 2007 Hygiene bei immunsuprimierten Patienten – presentation by Dr. A. Simon: page 3: MedicCleanAir® table with results
- 3 b) DGKH Germany 2005 – summary study Uni Bonn Germany – Dr. A. Simon
- 3 c) DGKH Germany 2006 – German organization for Hospital Hygiene: see page 9/24 right hand side, Monday April 3rd 2006, Workshop and discussions MedicCleanAir® from 1745-1900 Uhr – Saal 2
Conclusion
The University Hospital of Bonn came to the conclusion that they require 2 MedicCleanAir® Mobile HEPA Units when they want to bring the level of contamination down to zero (ZERO CFU/M3) in a (cancer) patient’s room, EVEN WITH OPEN WINDOWS. Units were operating at level 2, although the Units have the capability of giving more then double the capacity at level 4.
Report University Hospital Sart Tilman Liège Belgium – Infection Control Department – see attached letters regarding this scientific test
This University Hospital wanted to know how fast and with how much % MedicCleanAir® Mobile HEPA Units could reduce the existing contamination.
The following documents can be obtained at the office:
- 4 a) CHULiège051010Evaluation MedicCleanAir®-département hygiène: report from hospital
- 4 b) CHULiège051011rapport tests: final evaluation
Drawing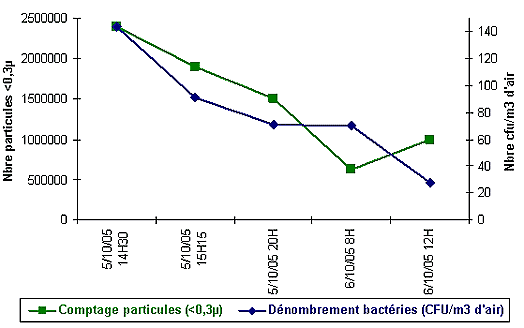
Conclusion
MedicCleanAir® Mobile HEPA Units reduce the contamination with minimum 75% to 100% as from a few minutes, continuously day in day out.
This organization has tested MedicCleanAir®
Mobile HEPA Units and have come to the following conclusion:
MedicCleanAir® Mobile HEPA Units are able to reduce the contamination of a room with size 3 x 5 x 3 meters height within maximum 30 minutes.
Drawing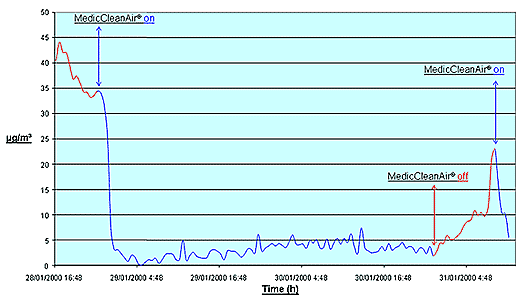
Conclusion
The measuring device of the organization was only able to measure the reduction of the contamination by the MedicCleanAir® units every 30 minutes. As from the first measuring point, contamination was already brought down to practically ZERO. Time lap in between the MedicCleanAir® devices are able to reduce contamination is therefore between 0 and 30 minutes.
Institut Pasteur was asked by the University Hospital St. Pierre Brussels for testing several purification units (MedicCleanAir® installed the units in 30 isolation rooms for infectious patients / Tuberculosis)
This Pasteur-organization is THE organization in France in relation to trustworthy testing of Mobile HEPA Units for medical facilities.
MedicCleanAir® Mobile HEPA Units are able to reduce the contamination of a room with size 3.46 x 4.70 x 2.70 height meters within 7 minutes when put at level 2. Level 2 was the level that we recommended as manufacturer.
Conclusion
MedicCleanAir® Mobile HEPA Units are able to reduce the contamination within 7.
Notre Dame des Bruyères – Operating Theatres
This test was done on particles as well as on Colony Forming Units/Bacteria
MedicCleanAir® first installed 2 units in the O.T. and afterwards 1 ISO unit to create positive pressure. The hospital did not change any other settings of existing ventilation, HVAC or air conditioning devices (although we requested to clean these thoroughly)
Results with MedicCleanAir® Units
Particle counts reduced from 8.297.495/m3 to 112.190/m3 (99% reduction)
CFU counts reduced from 31 CFU to 0 CFU (100% efficiency result)
The operating theatre was (without any other additional measures or changes other then MedicCleanAir®) re-classified to ISO 7.
Shahid-Rajai Cardiovascular Medical Center, Iran, University of Medical Sciences: operating theatre
This test to particle readings in one of the operating theatres. There were 4 professors involved, including the Dean of the Surgery Center. There was only 1 MedicCleanAir® Unit installed in the operating theatre and the readings were taken after only 10 minutes. Although this operating time is too short to take readings, the results are clear:
Results with MedicCleanAir® Units
Average reduction of 80% over all sizes of particles: 0.3 – 0.5 – 0.7 – 1.0 – 2.0 – 5.0 µm
The MedicCleanAir® Unit was placed in the ICU next to the operating theatre. The ICU is equipped with an existing Air Conditioning unit, taking air from outside the building and blowing it into the ICU without significant pré-filtration. (no integrated HVAC).
Results with MedicCleanAir® Units
Reductions because of MedicCleanAir®:
Everywhere to ZERO CFU (0) except on their instruments.
The MedicCleanAir® Unit was placed in Operating Theatre nr. 2. – only one unit although MedicCleanAir® advises always 2 units inside operating theatres.
The laminar flow system showed to contaminate the operating theatre severely. Although requested, no additional cleaning was performed, no change it normal ventilation settings.
Results with MedicCleanAir® Units
Reduction between 60 to 80% for particles of 0.5 micron to 5 micron – Classification of the Operating Theatre with MedicCleanAir® Units: ISO 6
General information
Supporting technical organizations
Filtec Prüflabor FFL AG – Switzerland
V.I.T.O - Belgo Nuclear – Belgium – Head of Laboratory
University of Leuven – Belgium – Prof. Dr. Bart Nicolaï
MedicCleanAir® – Belgium & Switzerland
Supporting medical organizations
Organizations that have published clinical, medical or/and scientifically studies performed with MedicCleanAir® Mobile HEPA Units:
Published in The Hospital Infection Society 2000
Published in The European Bone Marrow Transplant 2002
Presented at the International Congress of the German Institute for Hospital Hygiene 2006 (DGKH)
Published by the National Cancer Institute Italy 2002
Published in the Magazine of the National Institute for Tuberculosis Belgium 2005
Presented at the European Congress for clinical Microbiology and Infectious Diseases / Stockholm 2000
Presented at the International Congress for Pediatrics and Immunology / Brussels 2000
Published in Noso Info Belgium – Belgian Infection Control Society 2000
Published in the Epidemiologisch Bulletin van de Vlaamse Gemeenschap 2000
In Short
More technical security and medical support cannot be given
As stated by CDC as well, the only reliable sources that one has to support the decisions in relation to infection control guidelines are the multiple medical studies and technical organizations that prove that a certain device is effective and efficient. All other statements and brochures of manufacturers are nil, void and give no certainty at all.
We trust that the above information give you satisfaction regarding the capability and efficiency of our MedicCleanAir® Mobile HEPA Units.
«With the recent outbreak of the deadly Corona virus, it is extremely important for hospitals with isolated infected patients to keep the air in their environment safe and clean. Here is a small appearance of one of our air purification systems reported in the Belgian press this week.»







